Topic: Properties Of Water
Properties Of Water
During which phase change does water release the most heat energy?
(1) freezing
(2) melting
(3) condensation
(4) vaporization
Which change in the heat energy content of water occurs when water changes phase from a liquid to a solid?
(1) gain of 334 Joules of heat energy per gram
(2) release of 334 Joules of heat energy per gram
(3) gain of 2260 Joules of heat energy per gram
(4) release of 2260 Joules of heat energy per gram
During the process of condensation, water vapor
(1) releases 334 J/g of heat energy
(2) releases 2260 J/g of heat energy
(3) gains 334 J/g of heat energy
(4) gains 2260 J/g of heat energy
Which process releases 2260 joules of heat energy per gram of water into the environment?
(1) melting
(2) freezing
(3) condensation
(4) evaporation
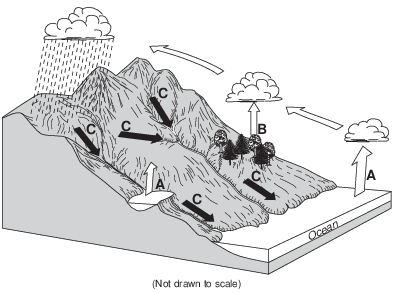
In order for process A to occur, liquid water must
(1) gain 334 Joules per gram
(2) gain 2260 Joules per gram
(3) lose 334 Joules per gram
(4) lose 2260 Joules per gram
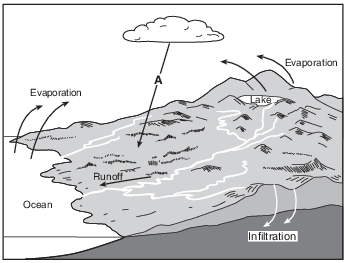
How many joules of heat energy are required to evaporate 2 grams of water from the lake surface? [1]
J
Allow 1 credit for 4520 J.
